
 Many in the field of nutrition have lost sight of the fact that there are two essential fatty acids needed by the body. Many people recommend omega-3 fatty acids assuming the the body gets sufficient omega-6 from the diet. The truth about essential fatty acids is more complicated. This article will show the more complete and correct picture.
Many in the field of nutrition have lost sight of the fact that there are two essential fatty acids needed by the body. Many people recommend omega-3 fatty acids assuming the the body gets sufficient omega-6 from the diet. The truth about essential fatty acids is more complicated. This article will show the more complete and correct picture.
BACKGROUND
Fatty acids are part of the lipids class, widely found in nature, food, and organisms. These fatty acids are a critical constituent of the cell membranes in all of the trillions of cells in the body. They have important biological functions including structural, communication, and metabolic roles, and they represent an important source of energy. Their metabolism produces a huge quantity of adenosine triphosphate (ATP). The beta-oxidation of the fatty acids is a well-known process, mostly used by the heart and the muscular tissue to obtain energy.
Figure 1 below shows a schematic diagram of what a fatty acid looks like. One end of the structure in all cases has a carboxylic acid group (COOH) and the other end in all cases has a methyl group (CH3). Saturated fats have single bonds (-) between all carbon atoms (C), but unsaturated fats have a number of double bonds (=) between some of the carbon atoms.
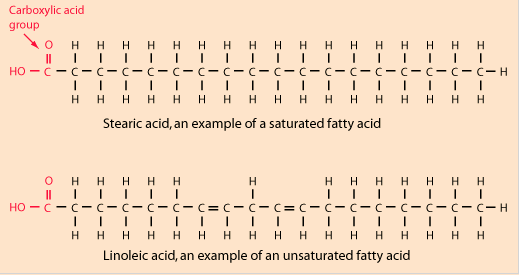
The human body can synthesize many of these fatty acids, except the essential fatty acids (PUFAs) linoleic acid (LA) and alpha-linolenic acid (ALA). These two are generally found in various vegetable oils, but their important metabolites are found mainly in special vegetable oils such as borage oil and in fish oils. Linoleic acid is the most abundant fatty acid in nature, and it is the precursor of other omega-6 fatty acids. Omega-3 fatty acids are synthesized from alpha-linolenic acid.
Once ingested, short-chain PUFAs are converted to long-chain fatty acids. These are critical for mammalian cells in order to perform various biological functions, such as sustaining the structural integrity of cellular membranes and serving as signaling molecules. They are highly enriched in brain tissues, where they participate in the development and maintenance of the central nervous system during both embryonic and adult stages.
Polyunsaturated fatty acids have been extensively researched. They include the essential fatty acids linoleic acid (an omega-6) and alpha linolenic acid (an omega-3). Omega-3s are not abundant in our food chain. There is none in corn oil and very little in soy oil, the two most widely used food oils. Therefore, nearly all the early research with polyunsaturated oils utilized omega-6 fatty acids, predominantly as linoleic acid.
Fish oils were neglected out of ignorance or because the investigators chose to pass over these cholesterol-containing oils. Concern eventually developed over the close association between increasing incidence of mammary tumors and high intake of omega-6 polyunsaturated fatty acids. After some years, researchers finally turned their investigations to the interrelationship between dietary omega-6 and omega-3 fatty acids.
FATTY ACID METABOLIC PATHWAYS
The following diagram shows in detail the pathways for the production and use of fatty acids in the body. In the figure the metabolic pathways (running left to right) for four fatty acids types are shown (top – Omega-3, second – Omega-6, third – Omega-9, bottom – Omega-7). Notice that only the omega-3 and omega-6 oils are considered to be essential fatty acids because they cannot be made in the body. This means they must come from food.
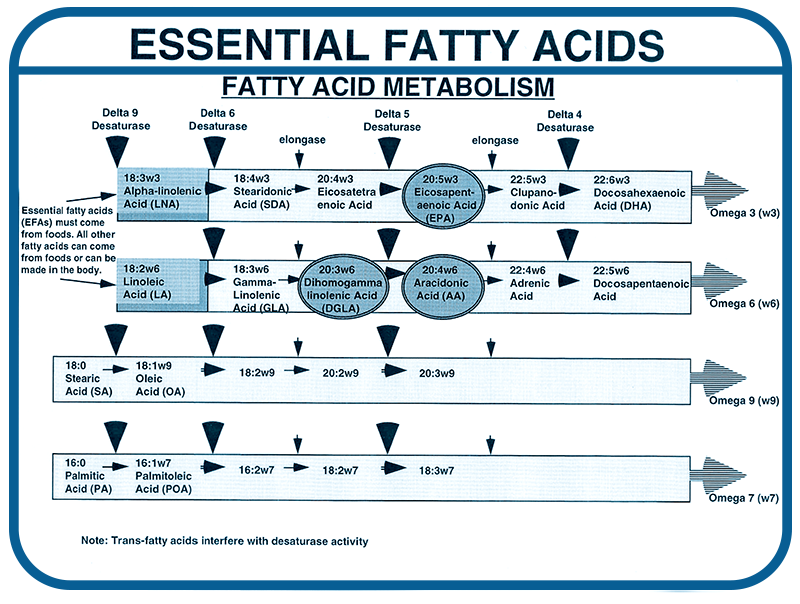 The diagram shows a series of enzyme induced reactions that either add a double bond or two additional carbon/hydrogen pairs to the fatty acid. The enzymes that make this happen are called desaturase and elongase. The desaturase enzymes are given a number for the carbon number (that the enzyme is working on) from the methyl end of the fat. These same enzymes work on all of the fatty acid types. For example, Delta 6 desaturase causes an additional double bond to be inserted into both alpha-linolenic (omega-3) and linoleic acid (omega-6) (as well as oleic acid and palmitoleic acids).
The diagram shows a series of enzyme induced reactions that either add a double bond or two additional carbon/hydrogen pairs to the fatty acid. The enzymes that make this happen are called desaturase and elongase. The desaturase enzymes are given a number for the carbon number (that the enzyme is working on) from the methyl end of the fat. These same enzymes work on all of the fatty acid types. For example, Delta 6 desaturase causes an additional double bond to be inserted into both alpha-linolenic (omega-3) and linoleic acid (omega-6) (as well as oleic acid and palmitoleic acids).
In this way, the body is able to produce a wide variety of fatty acids that have their own unique effects on biochemistry. Some of these are more important than others. In particular, the omega-3 essential fatty acid eicosapentanoic acid (EPA), the omega-6 essential fatty acid dihomo-gamma-linolenic acid (DGLA), and the omega-6 essential fatty acid arachidonic acid (AA) are precursors for a class of chemicals called eicosanoids/prostaglandins that have far reaching affects on key body functions.
EICOSANOIDS/PROSTAGLANDINS
Eicosanoids are prostaglandins that affect many aspects of health both positively and, in some cases, negatively. All known eicosanoids and prostaglandins are formed from the essential fatty acids linoleic acid (omega-6, or n-6), alpha linolenic acid (omega-3, or n-3), their “enhanced” derivatives, and from the omega-3 fatty acids in fish oils.
Prostaglandins are short-lived highly active, hormone-like chemicals that are found in every cell of the body. They are regulators of cell activity and essential for maintaining health. Each cell type or organ produces its own form of prostaglandin to carry out its functions. There are three types of prostaglandins: PG1, PG2, and PG3.
Series 1 Prostaglandins (PG1), derived from gamma-linolenic acid (GLA), the active component of borage oil, has many beneficial effects: It makes platelets less sticky, lowers blood pressure by relaxing smooth muscles in the walls of arteries, increases loss of sodium and water, decreases inflammation and enhances immunity.
Series 2 Prostaglandins (PG2), also derived from GLA, is used in “fight or flight” (stress) situations, – the fight against danger, or the flight from it. In modern lifestyles which are high in stress but low in physical activity, continuous production of Series Two Prostaglandins results in sticky platelets, high blood pressure, increased water and sodium retention, increased inflammation and decreased immune system capabilities.
Series 3 Prostaglandins (PG3), derived from eicosapentaenoic acid (EPA), the active component of fish oil, has beneficial effects. They block the detrimental effect of the Series 2 Prostaglandins, preventing them from being made in the body. As a result the platelets are less sticky, blood pressure is lower because the muscles in the walls of our arteries remain relaxed, loss of sodium and water by the kidneys takes place more effectively, inflammation response is decreased, and immune function is efficient.
It is now known that the ratios of these dietary fatty acids are very important. Consumption of linoleic acid leads to production of the enhanced fatty acid, arachidonic acid (20:4n-6). Prostaglandins based on arachidonic acid exacerbate stress and inflammatory states, and suppress immunoprotective functions (i.e. resistance to disease). Too much linolenic acid and other omega-3s may cause excessive bleeding during injury, surgery, or childbirth. Large amounts of any of these unsaturated fatty acids in the diet without a compensatory increase in antioxidant nutrients (especially Vitamin E), can speed oxidative damage to tissues, resulting in accelerated aging while increasing the risk of degenerative diseases.
Yet, a balanced ratio of both omega-3 and omega-6 fatty acids in the diet offers very positive health benefits. When omega-3 fatty acids predominate, the body will produce less arachidonic acid (20:4n-6). Immunity improves and inflammation subsides.

Unfortunately, our Western diet has been almost devoid of omega-3 fatty acids. Creating the optimum intake of omega 3-to-omega 6 unsaturated fatty acids has become, therefore, an issue of prime importance for anyone concerned with health. We need to evaluate carefully the amounts of linoleic acid (n-6) we consume relative to our intake of alpha-linolenic acid (18:3n-3) and fish oils (EPA:20:5n-3 and DHA:22:6n-3).
ESSENTIAL FATTY ACIDS – PATHWAYS
The diagram in Figure 3 shows details of the omega-6 and omega-3 pathways. Pathway specifics indicate key eicosanoids (series 1 prostaglandins, series 2 prostaglandins, and series 3 prostaglandins), oil sources, and important nutrient cofactors that are needed for the reactions to take place.
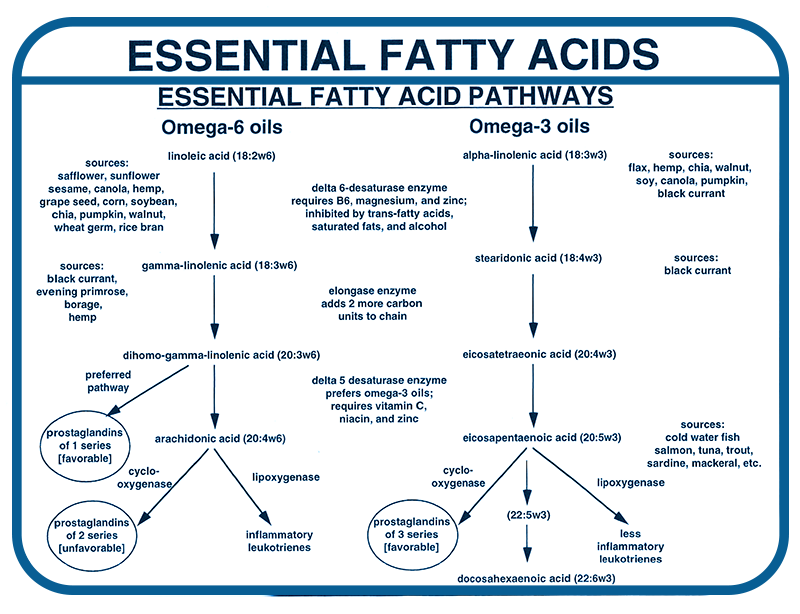
The information is this diagram gives the clues we need in order to provide optimal types and amounts of omega-6 and omega-3. For example, I have chosen for my essential fatty acid product cold pressed borage oil as the best natural source of gamma linoleic acid (GLA). It contains 20% by weight — the highest amount found in natural oils.
RESEARCH ON ESSENTIAL FATTY ACIDS
Work by Chapkin et. al. (see references 1–4 below) has identified the potent synergistic relationship between GLA, an omega-6 fatty acid, and the well-known omega-3 fatty acids. Chapkin has shown that, rather than simply the quantity of dietary omega-3s, it is the ratio of omega-6 to omega-3 fatty acids that is important in achieving full cardiovascular health and inflammatory control.
Furthermore, Chapkin has identified the ideal ratio. His published work deals with the importance of mixed diets supplying both linoleic and linolenic acids. To underscore the importance of these two fatty acids, refined oil supplements rich in enhanced forms were used. “Enhanced forms” are fatty acids derived from the original. They are one or more steps closer to the actual eicosanoid. In the human body, alpha linolenic acid (18:3n-3) is eventually converted to eicosapentaenoic acid (EPA, 20:5n-3) and linoleic acid (18:2n-6) is converted to gamma-linolenic (GLA, 18:3n-6) as its first enhanced form. Both enhanced fatty acids are precursors to eicosanoids.
In Chapkin’s research, superior health benefits were delivered by the mixed diet that supplied the eicosanoid precursors in a specific ratio. The balanced ratio of enhanced Omega-6 (GLA)-to-Omega-3 (EPA) fatty acids was 1:4.
IMPLEMENTATION OF THE SCIENCE
Based upon the science discussed above, I developed a product with the correct Omega-6 (GLA)-to-Omega-3 (EPA) ratio and with proper amounts. It is available to you as Hank & Brians Essential Fats Plus E from Health Products Distributors, Inc.

ESSENTIAL FATS PLUS E IS A HIGHLY ADVANCED ESSENTIAL FATTY ACIDS SUPPLEMENT
OFFERING SPECIAL BENEFITS:
-
UNIQUE COMBINATION — Essential Fats (EPA, DHA, GLA) plus Vitamin E. This unique formula offers more than one type of Vitamin E (not just d-alpha-tocopherol) and balanced essential fats.
-
BALANCED ESSENTIAL FATS— Many EFA supplements contain only omega-3s, but for optimal function the body requires a balance of omega-3 and omega-6 essential fats. In addition, our special formula provides a 4-to-1 ratio of EPA to GLA in order to achieve a balance you need for optimal health.
-
FULL-SPECTRUM VITAMIN E — Tocotrienols and tocopherols in this formula are natural vitamin E substances derived from oryza rice bran oil and protect polyunsatured EFAs against free-radical damage both in the capsule and in your body. Many Vitamin E supplements contain only d-alpha tocopherol, which is only a single component of the full-spectrum Vitamin E in this formula.
-
ULTRAPURE — Molecularly distilled oils of extremely high-purity containing no PCBs, heavy metals, or oxidized contaminants. Free of excipients, additives, and common food allergens!
COMPOSITION: Six softgel capsules provides the following percentages of the Daily Value.
| NUTRIENT | AMOUNT | % Daily Value† |
|---|---|---|
| EPA (Eicosapentaenoic Acid 20:5 omega 3) (from 2,000 mg of purified fish oils) |
360 mg | * |
| DHA (docosahexaenoic Acid 22:6 omega 3) (from 2,000 mg of purified fish oils) |
240 mg | * |
| GLA (Gamma Linolenic Acid 18:3 omega 6) (from 450 mg of cold pressed borage seed oil) |
90 mg | * |
| Vitamin E (d-alpha-tocopherol) (from 180 mg of Oryza rice bran oil) | 24 IU | 81% |
| Mixed Tocotrienols (d-gamma, d-alpha, and d-delta) (from 180 mg of Oryza rice bran oil) |
28.8 mg | * |
* No established Daily Value
† Daily Values based on a 2,000 calorie diet
IMPORTANT FUNCTIONS OF ESSENTIAL FATTY ACIDS
Below we provide some of the functions and benefits obtained when by diet or supplementation the correct ratios and amounts of essential fatty acids are consumed.
• Regulate steroid production and hormone synthesis
• Regulate pressure in the eyes, joints, and blood vessels
• Regulate response to pain, inflammation, and swelling
• Mediate Immune Response
• Regulate bodily secretions and their viscosity
• Dilate or constrict blood vessels
• Regulate smooth muscle and autonomic reflexes
• Are primary constituents of cellular membranes
• Regulate the rate at which cells divide
• Necessary for the transport of oxygen from the red blood cells to tissues
• Necessary for proper kidney function and fluid balance
• Prevent red blood cells from clumping together
• Regulate nerve transmission
GENETIC TESTING AND ESSENTIAL FATTY ACIDS
Please note that genetic testing for a wide range of genes and the enzymes they produce has indicated that essential fatty acids can be an important factor in helping the body overcome a variety negative gene variations. These negative gene variations include genes that relate to: 1) Inflammatory Response, 2) Exercise Performance, 3) Exercise Recovery, 4) Cardiovascular Fitness, 5) Body Composition, and 6) VO2 Max, Aerobic Capacity.
We will discuss this more deeply in a future blog article.
CONCLUSION
The body is best protected from a range of health issues when we supply a mixed diet of both omega-3 and omega-6 essential fatty acids. Studies show that we do not need to consume large amounts of fatty acids if the ratio is correct. These findings indicate that, for a typical human body, amounts of 90 mg GLA (18:3n-6) to 360 mg EPA (20:5n-3) taken daily will provide for the optimum production of the three major prostaglandins. These amounts are found in Hank & Brians Essential Fats Plus E.
REFERENCES
The following includes abstracts of Chapkin’s published research on essential fatty acids.
REFERENCE 1
Chapkin RS Somers SD Erickson KL
In: Lipids (1988 Aug) 23(8):766-70
Because alterations in the dietary content of fatty acids are an important method for modulating macrophage eicosanoid production, we have quantitated the levels of n-6 and n-3 polyunsaturated fatty acids in peritoneal macrophage individual phospholipids from mice fed diets (3 wk) with either safflower oil (SAF), predominantly containing 18:2n-6, borage, (BOR) containing 18:2n-6 and 18:3n-6, fish (MFO) containing 20:5n-3 and 22:6n-3, and borage/fish mixture (MIX) containing 18:2n-6, 18:3n-6, 20:5n-3 and 22:6n-3. Dietary n-3 fatty acids were readily incorporated into macrophage phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI). The increase in n-3 fatty acid levels was accompanied by a decrease in the absolute levels of 18:2n-6, 20:4n-6 and 22:4n-6 in PC, PE and PS. Interestingly, PI 20:4n-6 levels were not significantly lowered (P greater than 0.05) in MIX and MFO macrophages relative to SAF and BOR. These data demonstrate the unique ability of this phospholipid to selectively maintain its 20:4n-6 levels. In BOR and MIX animals, 20:3n-6 levels were significantly increased (P less than 0.05) in all phospholipids relative to SAF and MFO. The combination of borage and fish oils (MIX diet) produced the highest 20:3n-6/20:4n-6 ratio in all phospholipids. These data show that the macrophage eicosanoid precursor levels of 20:3n-6, 20:4n-6 and n-3 acids can be selectively manipulated through the use of specific dietary regimens. This is noteworthy because an increase in phospholipid levels of 20:3n-6 and 20:5n-3, while concomitantly reducing 20:4n-6, may have therapeutic potential in treating inflammatory disorders.
Institutional address: Department of Human Anatomy School of Medicine University of California Davis 95616.
REFERENCE 2
Chapkin RS Carmichael SL
In: Lipids (1990 Dec) 25(12):827-34
This study examined the effects of n-3 and n-6 polyunsaturated fatty acid alimentation on murine peritoneal macrophage phospholipids. Mice were fed complete diets supplemented with either corn oil predominantly containing 18:2n-6, borage oil containing 18:2n-6 and 18:3n-6, fish/corn oil mixture containing 18:2n-6, 20:5n-3 and 22:6n-3, or fish/borage oil mixture containing 18:2n-6, 18:3n-6, 20:5n-3 and 22:6n-3. After two weeks, the fatty acid levels of glycerophosphoserines (GPS), glycerophosphoinositols (GPI), sphingomyelin (SPH), and of the glycerophosphocholine (GPC) and glycerophosphoethanolamine (GPE) phospholipid subclasses were determined. We found that mouse peritoneal macrophage GPC contain primarily 1-O-alkyl-2-acyl (range for the dietary groups, 24.6-30.5 mol %) and 1,2-diacyl (63.2-67.2 mol %), and that GPE contains 1-O- alk-1′-enyl-2-acyl (40.9-47.4 mol %) and 1,2-diacyl (44.2-51.2 mol %) subclasses. In general, fish oil feeding increased macrophage 20:5n-3, 22:5n-3 and 22:6n-3 levels while simultaneously reducing 20:4n-6 in GPS, GPI, GPE and GPC subclasses except for 1-O-alk-1′-enyl-2-acyl GPC. Administration of 18:3n-6 rich diets (borage and fish/borage mixture) resulted in the accumulation of 20:3n-6 (2-carbon elongation product of 18:3n-6) in most phospholipids. In general, the novel combination of dietary 18:3n-6 and n-3 PUFA produced the highest 20:3n-6/20:4n-6 phospholipid fatty acid ratios. This study demonstrates that marked differences in the responses of macrophage phospholipid classes and subclasses exist following dietary manipulation.
REFERENCE 3
Fan YY Chapkin RS
Mouse peritoneal macrophage prostaglandin E1 synthesis is altered by dietary gamma-linolenic acid.
In: J Nutr (1992 Aug) 122(8):1600-6
The ability of dietary gamma-linolenic acid [18:3(n-6)] to modulate prostaglandin biosynthesis in mouse resident peritoneal macrophages was determined. Mice were fed diets containing corn oil, borage oil or evening primrose oil or a mixture of borage and fish oils. After 2 wk, resident peritoneal macrophages were isolated and stimulated with unopsonized zymosan to induce prostaglandin synthesis. Borage oil, primrose oil and fish-borage oil mixture dietary groups (containing 25.6, 11.9 and 19.5 g gamma-linolenic acid/100 g fatty acids, respectively) had significantly (P less than 0.05) enhanced prostaglandin E1 synthesis (39.7, 29.4 and 73.0 nmol prostaglandin E1/mg protein, respectively) compared with corn oil-fed (containing less than 0.1 g gamma-linolenic acid/100 g fatty acids) animals, which synthesized less than 0.1 nmol prostaglandin E1/mg protein. Borage oil- and fish-borage oil mixture-fed mice had the highest biosynthetic ratio of prostaglandin E1/prostaglandin E2 (E1/E2 approximately 0.2). Macrophages from borage oil-fed mice synthesized the lowest amount of prostacyclin (198.7 nmol 6-keto-prostaglandin F1 alpha/mg protein) compared with corn oil-, primrose oil- and fish- borage oil mixture-fed mice (379.7, 764.8 and 384.2 nmol 6-keto- prostaglandin F1 alpha/mg protein, respectively). In addition, borage oil-, primrose oil- and fish-borage oil mixture-fed mice had significantly (P less than 0.05) higher levels of dihomo-gamma- linolenic acid [20:3(n-6)] in membrane phospholipids (5.5, 3.5 and 5.7 mol/100 mol, respectively) relative to corn oil-fed mice (2.0 mol/100 mol).
REFERENCE 4
Fan YY Chapkin RS Ramos KS
Dietary lipid source alters murine macrophage/vascular smooth muscle cell interactions in vitro.
In: J Nutr (1996 Sep) 126(9):2083-8
This study was conducted to compare the impact of dietary lipids on the ability of macrophages to modulate vascular smooth muscle cell (SMC) DNA synthesis in vitro. C57BL/6 female mice were fed six different diets (6 mice/diet) containing 10% fat from corn oil (CO), borage oil (BO), primrose oil (PO), fish-corn oil mix (FC, 9:1, w/w), fish-borage oil mix (FB, 1:3, w/w), or fish-primrose oil mix (FP, 1:3, w/w) for 2 wk. Peritoneal macrophages were isolated from these mice, stimulated with zymosan or vehicle, and subsequently co-cultured with naive mouse aortic SMC in the presence of 3H-thymidine to measure SMC DNA synthesis. In this co-culture system, macrophages were seeded on 25-mm culture inserts (upper chamber) and SMC were seeded on 35-mm culture dishes (lower chamber). The two cell types were separated by a semipermeable membrane with a 30-kD cut-off. When quiescent SMC were co-cultured with macrophages, only the PO and FP diet groups had significantly (P < 0.05) lower SMC DNA synthesis compared with the control CO group whose diet contained no gamma- linolenic acid (GLA) or (n-3) polyunsaturated fatty acids (PUFA). In contrast, when cycling SMC were co-cultured with diet-modulated macrophages, all dietary groups except for those fed FC had significantly lower (P < 0.05) SMC DNA synthesis relative to the CO group. Although the level of GLA in PO and BO diets was different (11.5 and 22.3 g/100 g fatty acids, respectively), these treatments exerted comparable inhibitory effects on SMC DNA synthesis. The FP treatment consistently exhibited the lowest SMC DNA synthetic profile among the six dietary groups irrespective of SMC growth conditions. These data suggest that BO and PO alone or in combination with fish oil influence macrophage/smooth muscle cell interactions in a manner consistent with favorable modulation of the atherogenic process.
These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure or prevent any disease.
BOOKS
- Enig, Mary G. Know Your Fats: The Complete Primer for Understanding the Nutrition of Fats, Oils, and Cholesterol. Bethesda Press, 2000.

